
TECHNICAL SPECIFICATIONS
Agilent 6850 GC
- Max 1 capillary column.
- ID column: 0.050 to 0.530mm.
- Detector: TCD, Tmax=350°C.
- Injector: cool on-column, T from RT to 300°C, or programmable T and track-on to follow the oven T.
- Fully programmable carrier flow (He).
- Data station: Agilent software on PC
AVAILABLE TECHNIQUES
Synthesis of the catalysts
- The laboratories of University Department are fully equipped for synthesis and purification both in air either in inert atmosphere (Argon), as well as for the characterization of the complexes by instrumental techniques such as Multinuclear NMR, ESI-MS, UV-VIS, IR, X-Ray diffractometers and elemental analysis.
Quantitative and qualitatives analysis of the reaction mixtures
- In the laboratory 109B a capillary Gas-Chromatograph Agilent 6850 is available for the quantitative analysis; such GC is equipped with a TCD detector and a cool on-column injector, a configuration particularly well suited for direct quantitative analysis of complex reactions mixtures, with high precision and without needing of pre-separation of the catalyst solved in the reaction medium. Otherwise, a qualitative and quantitative analysis can be performed in the University labs by techniques as NMR or GC-MS, which allow a full identification of the components of the reaction mixture besides their quantification.
Case Studies
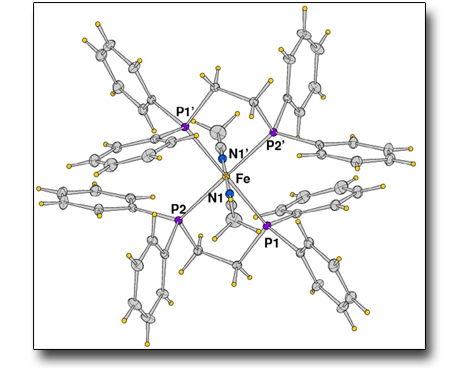
Iron complexes with polydentate phosphines as unusual catalysts for alcohol oxidation
Use of phosphinic ligands for oxidation catalysis is quite rare since themselves are easily oxidized during the reaction. In this report we show that these specific compounds are efficient to promote the oxidation of both secondary alcohols and glycerol by peroxides. The structure of some of these catalysts has been determined by X-Ray diffraction and we carried out investigations by NMR, UV-VIS and ESI-MS to clarify the true nature of the calysts and the reaction mechanism.
See: E. Farnetti et al., Inorg. Chim. Acta. 502, 119318 (2020)
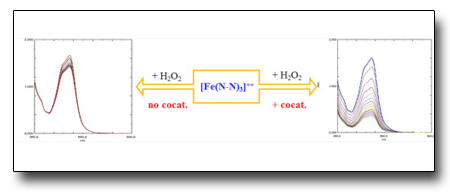
Microwave-assisted green oxidation of alcohols with hydrogen peroxide catalyzed by iron complexes with nitrogen ligands.
We investigated some Iron complexes with chelating nitrogen ligands, applied to promote oxidation of secondary alcohols by peroxides. The reaction have been carried out with traditional heating system as well as by microwave reactor, comparing advantages and features of both systems. The evolution of the catalytic system has been monitored by UV-VIS spectroscopy.
See: I.S.Cozzi et al., J. Organomet. Chem. 878, 38 (2018)
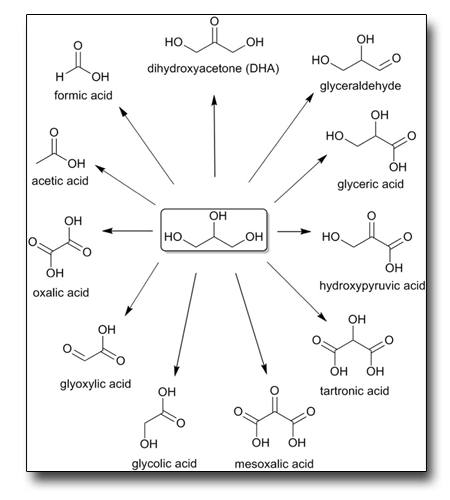
Selective oxidation of glycerol to formic acid catalyzed by iron salts
Glycerol is the main by-product in the bio-fuels industry and its valorization would improve the economicity of such route as an alternative to the fossil fuels. We have identified a simple transformation of glycerol by hydrogen peroxide catalyzed by Iron salts to produce selectively formic acid, itself a well known valid intermediate for the hydrogen productio.
See: E.Farnetti et al., Catalysis Communication 84, 1 (2016)

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)